TOLERANCE DEFINITION
Tolerance is specific immunologic unresponsiveness. In other words, it is a condition when an immune response to a certain antigen or epitope does not occur, the immune system will otherwise function normally. Although both B cells and T cells participate
AUTOIMMUNITY DEFINTION
When a host’s immune system focuses its attack on the host instead of reacting against foreign antigens, the condition can result in a clinical syndrome referred to as autoimmunity. It results from some failure of the host’s immune system to distinguish self from nonself.
Autoimmune reactions can cause serious damage to cells and organs, sometimes with fatal consequences. In some cases, the damage to self cells or organs is a result of antibodies. While in others, T cells are the culprit.
Lets study each topic one by one in detail…
TOLERANCE
As I mentioned earlier, tolerance or ‘self-tolerance‘ is best defined as a state of antigen-specific immunological unresponsiveness. Establishing self-tolerance involves both the elimination of immune cells and active inhibition of immune responses.
The term tolerance applies to the many layers of protection imposed by the immune system to prevent the reaction of its cells and antibodies against host components. In other words, individuals should not typically respond aggressively against their own antigens, although they will respond to pathogens or even cells from another individual of the same species.
Types of Tolerance
Depending upon the state of induction, tolerance is of two types:
- Central tolerance
- Peripheral tolerance

Central tolerance
Central tolerance refers to the tolerance established by deleting autoreactive lymphocyte clones before they develop into fully immunocompetent cells. It is the main way the immune system learns to discriminate self from
Central tolerance occurs in the primary lymphoid organs: the bone marrow for B cells and the thymus for T cells.
Peripheral tolerance
It develops after T and B cells mature and enter the peripheral tissues and lymph nodes.
Peripheral tolerance is key to preventing over-reactivity of the immune system to various environmental entities such as allergens. Moreover, it is the backup precaution of the immune system as central tolerance is not perfect and some self-reactive lymphocytes find their way into the periphery and secondary lymphoid tissues.
Peripheral tolerance involves deleting, rendering anergic or actively suppressing escaped lymphocytes that possess receptors that react with
Induction of Tolerance
Whether an antigen will induce tolerance or not is largely determined by the following:
- The immunologic maturity of the host: neonatal animals are immunologically immature and do not respond well to foreign antigens; for instance, neonates will accept allografts that would be rejected by mature animals.
- The structure and dose of the antigen: a very simple molecule induces tolerance more readily than a complex one, and very high or very low doses of antigen may result in tolerance instead of an immune response. Purified polysaccharides or amino acid copolymers injected in very large doses result in “immune paralysis”—a lack of response.
Other aspects of the induction are as follows:
- T cells become tolerant more easily and remain tolerant longer than B cells.
- Administration of a cross-reacting antigen tends to terminate tolerance.
- Administration of immunosuppressive drugs enhances tolerance (e.g., in patients who have received organ transplants).
- Tolerance is maintained best if the antigen to which the immune system is tolerant continues to be present.
Mechanism of tolerance induction
The exact mechanism of induction of tolerance is not clear. However, there are several possibilities
Clonal Deletion
The main process by which T lymphocytes acquire the ability to distinguish self from nonself occurs in the fetal thymus. This process, called clonal deletion, involves the killing of T cells (“negative selection”) that react against antigens (primarily self major histocompatibility complex [MHC] proteins) present in the fetus at that time. The self-reactive cells die by a process of programmed cell death called apoptosis.
For negative selection and clonal deletion to be efficient, the thymic epithelial cells must display a vast repertoire of “self” proteins. A transcriptional regulator called the autoimmune regulator (AIRE) enhances the synthesis of this array of self proteins.
Clonal Anergy
It is the term used to describe self-reactive T cells that are not activated because proper costimulation does not occur. The mechanism of clonal anergy involves the inappropriate presentation of antigen, leading to a failure of interleukin-2 (IL-2) production. Inappropriate presentation is due to a failure of “costimulatory signals”.
Clonal Ignorance
It refers to self-reactive T cells that ignore self-antigens. These self-reactive T cells are either kept ignorant by physical separation from the target antigen or ignore self-antigens because the antigens are present in such small amounts.
Anti-ideotype antibody
These are antibodies that will produce against the specific idiotypes of other antibodies. Anti-idiotypic antibodies will produce during the process of tolerization and have been demonstrated in tolerant animals. These antibodies may prevent the B cell receptor from interacting with the antigen.
Termination of tolerance
Experimentally induced tolerance can be terminated by the prolonged absence of exposure to the tolerogen, by treatments which severely damage the immune system (x-irradiation) or by immunization with cross-reactive antigens.
Tolerance Summary
B-cell tolerance can result both from clonal anergy and clonal deletion, and the choice of the mechanism depends on whether the antigen is soluble or membrane-bound. Clonal deletion involves apoptosis of the self-reactive cells, but only membrane-bound antigens appear to trigger apoptosis. B-cell
AUTOIMMUNITY
It results from some failure of the host’s immune system to distinguish self from nonself. In other words, Autoimmune diseases occur due to the failure of the tolerance that protects the host from the action of self-reactive lymphocytes.
The most important step in the production of autoimmune disease is the activation of self-reactive helper (CD4) T cells. This self-reactive Th-1 or Th-2 cells can induce either cell-mediated or antibody-mediated autoimmune reactions, respectively.
Autoimmune disease affects between 3% and 8% of individuals in
Classification of auto-immune diseases
There are several different ways to classify autoimmune diseases. Several autoimmune diseases are strongly linked with MHC antigens. Therefore, autoimmune diseases are classified according to their association with class I or class II MHC markers (in most recent classification method).
- MHC class II-associated
- Organ-specific
- Non-organ specific (systemic)
- MHC class I–associated
- HLA-B27–related spondyloarthropathies (ankylosing spondylitis, Reiter’s syndrome, etc.) B.
- Psoriasis v
ulgaris (associated with HLA-B13, B16, and B17)
For more information: see Major histocompatibility complex
Organ-specific autoimmune diseases (MHC class II-associated)
In an organ-specific autoimmune disease, the immune response usually direct to a target antigen unique to a single organ or gland. The cells of the target organs may be damaged directly by humoral or cell-mediated effector mechanisms. Alternatively, anti-self antibodies may overstimulate or block the normal function of the target organ.
Examples: Type 1 Diabetes Mellitus, Hashimoto’s thyroiditis, etc.,
Non-organ specific
In systemic autoimmune diseases, the immune response is directed toward a broad range of target antigens and involves a number of organs and tissues. These diseases reflect a general defect in immune regulation that results in hyperactive T cells and/or B cells. Typically, tissue damage is widespread, both from cell-mediated immune responses to direct cellular damage.
Examples: Multiple Sclerosis, Rheumatoid Arthritis, etc.,

Factors affecting Autoimmune diseases
Both intrinsic and extrinsic factors favour the susceptibility to autoimmune diseases.
Environmental factors
Some autoimmune syndromes are more common in certain geographic locations or in particular climates. This suggests a link between environmental exposures or lifestyle factors, such as diet, in the development of the autoimmune disease. Similarly, there are several environmental agents that trigger autoimmune diseases, most of which are either bacteria or viruses. For example, pharyngitis caused by Streptococcus
Hormonal factor
Approximately 90% of all autoimmune diseases occur in women. Although the explanation for this markedly unequal gender ratio is unclear, there is some evidence from animal models that estrogen can alter the B-cell repertoire and enhance the formation of antibody to DNA.
Genetic factors
Certain alleles within the MHC have been linked to several different autoimmune disorders. There is a strong association of some diseases with certain human leukocyte antigen (HLA) specificities, especially the class II genes. For example, rheumatoid arthritis occurs predominantly in individuals carrying the HLA-DR4 gene. Similarly, Ankylosing spondylitis is 100 times more likely to occur in people who carry HLA-B27, a class I gene, than in those who do not carry that gene.
Mechanism for Autoimmunity
The following main mechanism for autoimmunity are as follows:
- Molecular Mimicry
- Alteration of Normal Proteins
- Release of Sequestered Antigens
- Epitope Spreading
- Failure of Regulatory T Cells
Molecular Mimicry
A number of viruses and bacteria possess antigenic determinants that are similar or even identical to normal host-cell components, led to a hypothesis known as molecular mimicry. This proposes that some pathogens express protein epitopes resembling self components in either conformation or primary sequence. For instance, molecular mimicry is seen in rheumatic fever. In this case, antibodies against certain M proteins cross-react with cardiac myosin which leads to heart damage, resulting in immune complex deposition and complement activation, a type II hypersensitivity reaction.
Alteration of Normal Proteins
Drugs can bind to normal proteins and make them immunogenic. Procainamide-induced systemic lupus erythematosus is an example of this mechanism.
Release of Sequestered Antigens
Certain tissues are sequestered so that their antigens are not exposed to the immune system. When such antigens enter the circulation accidentally (e.g., after damage), they elicit both humoral and cellular responses. Intracellular antigens, such as DNA, histones, and mitochondrial enzymes, will normally sequester from the immune system. However, bacterial or viral infection may damage cells and cause the release of these sequestered antigens, which then elicit an immune response.
In addition to infection, radiation and chemicals can also damage cells and release sequestered intracellular components. For example, sunlight may exacerbate the skin rash in patients with systemic lupus erythematosus.
Epitope Spreading
Epitope spreading is a term that describes the new exposure of sequestered autoantigens as a result of damage to cells caused by a viral infection. These newly exposed autoantigens stimulate autoreactive T cells and autoimmune disease results.
Failure of Regulatory T Cells
Regulatory T cells (Tr) suppress the proinflammatory effects of other T cells. An important function of Tr cells is to produce IL-10, which inhibits proinflammatory Th-1 cells. Patients with a mutation in the FoxP3 gene have an increase in autoimmune diseases, such as systemic lupus erythematosus, because they have lost the function of their regulatory T cells.
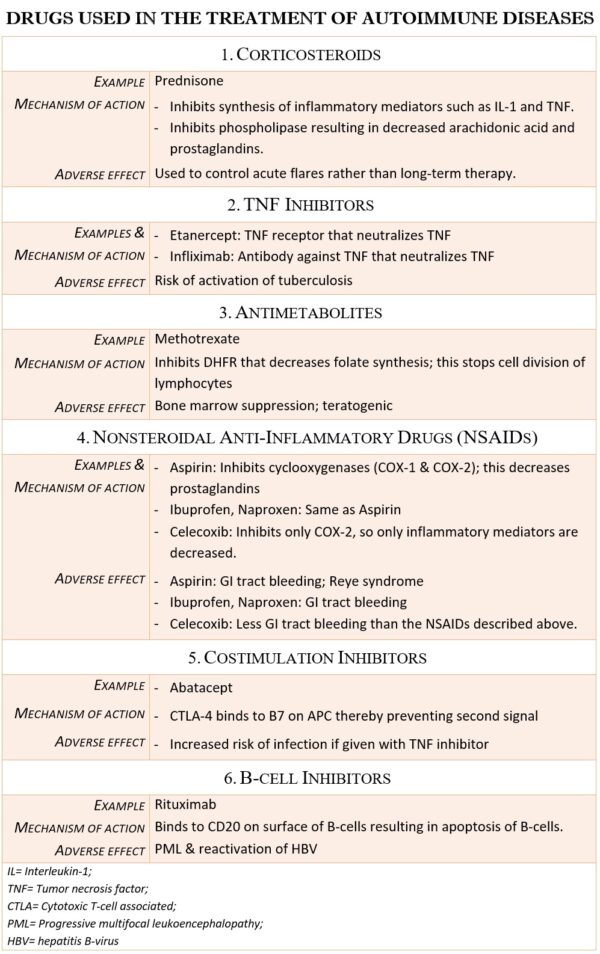
Treatment

The conceptual basis for the treatment of autoimmune diseases is to reduce the patient’s immune response or inflammatory response sufficiently to eliminate the symptoms. Ideally, treatment for autoimmune diseases should reduce only the autoimmune response, leaving the rest of the immune system intact. However, implementing this strategy has proven difficult. The current therapies to treat autoimmune disease fall into the following categories:
- broad-spectrum immunosuppressive treatments
- Strategies that Target Specific Cell Types
- Therapies that block steps in the inflammatory process
- Antigen-Specific Immunotherapy
Broad-spectrum immunosuppressive treatments
Most first-generation therapies for autoimmune diseases are not cures but merely palliatives, reducing symptoms to provide the patient with an acceptable quality of life. For example, most general immunosuppressive treatments (e.g., corticosteroids, azathioprine, and cyclophosphamide) are strong anti-inflammatory drugs that suppress lymphocytes by inhibiting their proliferation or by killing these rapidly dividing cells
Side effects
- general cytotoxicity,
- an increased risk of uncontrolled infection, and
- the development of cancer.
- Therapies That Block Steps in the Inflammatory Process
- strategies that interfere with costimulation
Strategies That Target Specific Cell Types
A monoclonal antibody against the B-cell-specific antigen CD20 (Rituximab) depletes a subset of B cells and provides short-term benefit for rheumatoid arthritis. However, most cell-type-specific agents used to treat autoimmune disorders target T cells or their products because these cells are either directly pathogenic or provide help to autoreactive B cells.
Therapies That Block Steps in the Inflammatory Process
Drugs that block TNF alpha, one of the early mediators in the inflammatory process, are widely used to treat Rheumatoid Arthritis, P
side effects
- increased risk of infections such as activating latent tuberculosis, skin and soft tissue infections etc.,
Strategies that interfere with costimulation
T cells require both antigenic stimulation via the TCR (signal 1) and costimulation (signal 2) in order to become fully activated. Without costimulation, T cells undergo apoptosis, become anergic, or induced as immune inhibitors. Therefore, one way to control T-cell activation would be to regulate costimulation.
Antigen-Specific Immunotherapy
The holy grail of immunotherapy to treat autoimmune disease is a strategy that specifically targets the autoreactive cells, sparing all other leukocytes. Glatiramer acetate is the only FDA-approved antigen-specific treatment currently available for autoimmune disease.
Sources and links
Kuby immunology, 7th edition, chapter no. 16
Review of medical microbiology and immunology by Warren Levinson, fourteenth edition, part VII; chapter no. 66
Medical immunology, fifth edition revised and expanded, edited by Gabriel Virella, part III: clinical immunology, chapter no. 16: Tolerance and Autoimmunity